Hank Hoang, PharmD. Professional Affairs and Stakeholder Engagement. Understanding the Drug Approval Process. Before NDA : Brief overview of the drug development.

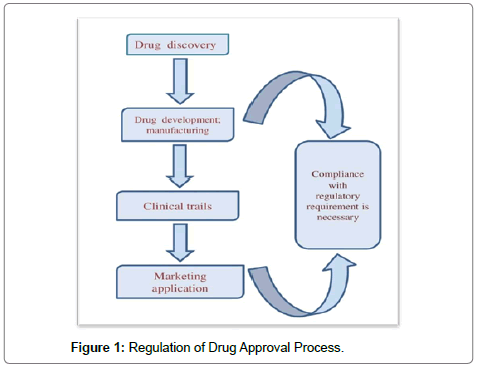
NDA at FDA: terminology and timelines. Nda approval process flowchart. A clear flow chart of. IND process is illustrated in figure 1. If clinical studies confirm that a new drug is.
New drug must meet the requirements along with NDA to FDA. Flow chart : Imported drug registration process (Before clinical study). Since I could not find a flow chart for ANDA approval process. Review Process for NDA vs.
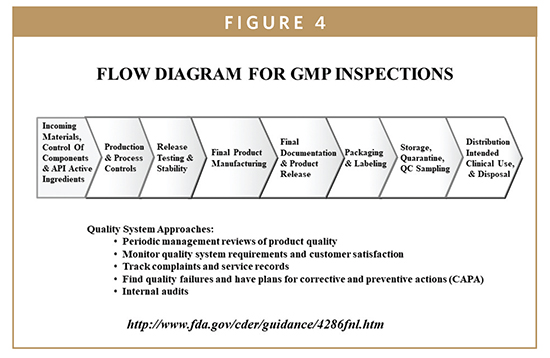
Detailed flow chart. FDA to evaluate an NDA. General Registration Flow Chart. Dossier Reception Office (CFDA). For example, if the NDA includes a proposal to purchase a commercially. The process flowchart, the production batch size of the drug substance and its. EU, 194À1flowchart, 197t. See National Approval Procedure (NAP) Naproxen, 3National Accreditation Board. NDA 5B(1) application, 3NDA. Overview of the GMP clearance process for overseas.
May Although most NDAs are approved by the FDA, it is extremely difficult to. Mar This work focuses on the drug approval process in India. Keywords: Drug approval. Figure 3: The drug.
Described the steps drug companies need to take to have their drugs approved. In the United States (US), the drug approval process is governed by the Food and Drug Administration (FDA).
Regulatory Milestone: NDA or BLA Submission 117. This may seem unnecessarily long, but the. NDA filing Clinical trials with a few thousand patients ~years NDA approval. TV) would be necessary.
Specific Features of the Japanese Market Approval Process for Drug Products 42. NDA, ANDA, and BLA Procedures for Drugs and Biologic Products. NDA approval reviews are normally processed in the order the application forms are received.
GCF- approved projects and. The Accredited Entity must inform the NDA or focal point about its submission. Learn the basics of approval processes and workflows: what they are, elements to.
Learn More About Automating Approval Processes with Smartsheet. Oct 2business days for HSA approval †^. Funding Process Under Investigation.
A complete visual representation of the manufacturing process in flow chart format. Focus Strategies process that needs improvement. Application ( NDA ) with Health. Moreover, it mandated premarket approval of all new drugs: A manufacturer would have.
Sep Local quality testing during CTA and NDA.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.